Lead Glass: Properties, Production, and Application
Lead glass is a glass variant which has had its calcium component replaced by lead. It contains between 18 to 40% of lead (II) oxide by weight. The nomenclature of lead glass is regulated such that any glass that contains a minimum of 24% PbO by weight is referred to as “lead crystal”, and any glass that contains less than 24% PbO by weight is referred to as crystal glass [1]. Lead glass has a chemical composition of SiO2 and PbO along with other impurities or additives added to achieve certain properties.
Lead glass is also known as X-ray glass or radiation shielding glass as one of its major applications is in the absorbance of high energy radiation while maintaining optical transparency. The presence and quantity of PbO are what determines some of lead glass’ most important optical and physical properties
In this article, you will learn about:
- The properties of lead glass
- The production of lead glass
- Applications of lead glass

Figure 1. Lead glass is used in microscope lenses due to its high refractive index.
Properties of lead glass
Lead glass, when compared to ordinary glass (also referred to as soda-glass or soda-lime silica-glass), is a dense and heavy material as lead is more than five times as heavy as calcium. Soda glass has a density of 2.4 g/cm3 and lead glass has a density of 3.1 - 5.9 g/cm3. Lead glass also has higher electrical resistance, higher refractive index (up to 1.8 compared to 1.5 for lead-free glass), lower working temperature and lower viscosity [2].
Lead glass is thus more fluid and easier to work with and fabricate than soda glass because it can be worked on at a wider temperature range while still being more malleable and less brittle than ordinary glass. Table 1 below shows a comparison of different lead glass materials in optical, mechanical, thermal, and electrical properties.
Table 1. Properties of selected types of commercial lead glasses
| SCHOTT 8095 | SCHOTT 8531 | SCHOTT 8532 | SCHOTT 8650 | SCHOTT 8651 | |
| Refractive index | 1.56 | 1.7 | 1.72 | 1.62 | 1.55 |
| Stress optical coefficient | 3.1*10-6 mm²/N | 2.2*10-6 mm²/N | 1.7*10-6 mm²/N | 2.8*10-6 mm²/N | 3.6*10-6 mm²/N |
| Elastic modulus | 60 GPa at 20 °C | 52 GPa at 20 °C | 56 GPa at 20 °C | 62 GPa at 20 °C | 59 GPa at 20 °C |
| Poisson's ratio | 0.22 at 20 °C | 0.24 at 20 °C | 0.24 at 20 °C | 0.23 at 20 °C | 0.24 at 20 °C |
| Loss tangent | 1.1*10-3 at 20 °C | 9*10-4 at 20 °C | 9*10-4 at 20 °C | 3.3*10-3 at 20 °C | 3.1*10-3 at 20 °C |
| Dielectric constant | 6.6 at 20 °C | 9.5 at 20 °C | 10.2 at 20 °C | 7.6 at 20 °C | 6 at 20 °C |
| Electrical resistivity | 3.98*107 Ω·m at 250 °C | 1.00*109 Ω·m at 250 °C | 1.00*109 Ω·m at 250 °C | - | 1.58*109 Ω·m at 250 °C |
| Coefficient of thermal expansion | 9.1*10-6 1/K at 20 °C | 9.1*10-6 1/K at 20 °C | 8.7*10-6 1/K at 20 °C | 5.1*10-6 1/K at 20 °C | 4.4*10-6 1/K at 20 °C |
| Specific electrical resistance temperature | 330 °C | 450 °C | 440 °C | - | - |
| Annealing temperature | 435 °C | 430 °C | 430 °C | 475 °C | 540 °C |
| Working temperature | 982 °C | 820 °C | 760 °C | 885 °C | 1034 °C |
| Glass transition temperature | 430 °C | 435 °C | 435 °C | 475 °C | 549 °C |
| Softening temperature | 630 °C | 585 °C | 560 °C | 625 °C | 736 °C |
| Thermal conductivity | 0.9 W/(m·K) at 90 °C | 0.7 W/(m·K) at 90 °C | 0.7 W/(m·K) at 90 °C | 0.5 W/(m·K) at 90 °C | 0.9 W/(m·K) at 90 °C |
| Corrosion properties | Hydrolytic resistance Class HGB3 (ISO 719), Acid resistance Class S2 (DIN 12116), Alkali resistance Class A3 (ISO695) | ||||
| Application areas | General electro-technical application and electro-technology (Signal intensifier, Detector and (fibre-) optics), Endoscopy. | Encapsulation of semiconductor components at low temperature (Micro-diodes), Electronics, Sensors | Encapsulation of semiconductor components at low temperature (Micro-diodes), Electronics, Sensors. | Implosion diodes, Electronics and Sensors | For power and pin diodes as well as micro-diodes, Signal intensifier, detector (other Electro-technology / Electronics), Sensors |
Production of lead glass
The production of lead glass generally proceeds as follows [3]:
- Lead oxide and/or lead tetroxide (PbO and/or Pb3O4) are fed into a silo.
- Other raw materials components (solid inorganic compounds that may be naturally occurring or synthetic) required to achieve the desired lead glass composition are mixed with lead material. A typical ratio of materials could include 48% silica-sand, 28% lead and 24% potash. Other metals may be added to the mix to give the glass a specific colour.
- The mixture is then fed into an electric furnace where it undergoes heat generated by its resistance to an electric current passing through it. Temperatures between 1350 °C and 1550 °C are required.
- Fresh batch material may be added to the melt, depending on the output requirements.
- The molten homogeneous glass melt is extracted and cooled slowly to avoid the development of residual stresses as different portions of the lead glass cool at different rates. The lead glass is produced as ribbons, droplets or gobs, which will be further shaped by forming techniques, such as pressing or blowing.
Applications of lead glass
The properties of lead glass make it useful for many applications. The majority of them, however, make use of its optical properties, in particular. Here are a few examples [3]:
- In the medical and research fields where visibility is required during procedures or experiments that involve radiation, lead glass can achieve the same radiation attenuation as the surrounding walls or barriers while allowing visibility to the operator. A typical example is the viewing window of an X-ray room or a laboratory experimenting with radioactive materials.
- The high refractive index of lead glass is useful for the production of lenses as thinner lenses can be crafted to achieve similar focal lengths to ordinary ophthalmic glass. Lead glass is thus used for special medical glasses, binoculars, microscopes and telescopes.
- Digital projectors use lead glass because it has a high level of transmission over short wavelengths, which is typical of projector laser beams. Further, the temperature rise caused by the light source does not distort the image projected by lead glass because it has a lower thermal conductivity than lead-free glass.
- It is useful in instruments that operate in the near-ultraviolet region and the near-infrared region as in bio-fluorescence, gene analyses, spectrometry and telecommunication.
- Lead glass is used in laser optics for printing and photocopying technologies.
- Lead glass used to be popular for its use as glassware, but due to the health implications of ingesting lead, its application for storing and serving drinks has dwindled dramatically in recent times. Instead, lead crystal is used for ornamental purposes, such as chandeliers and decorative vases.
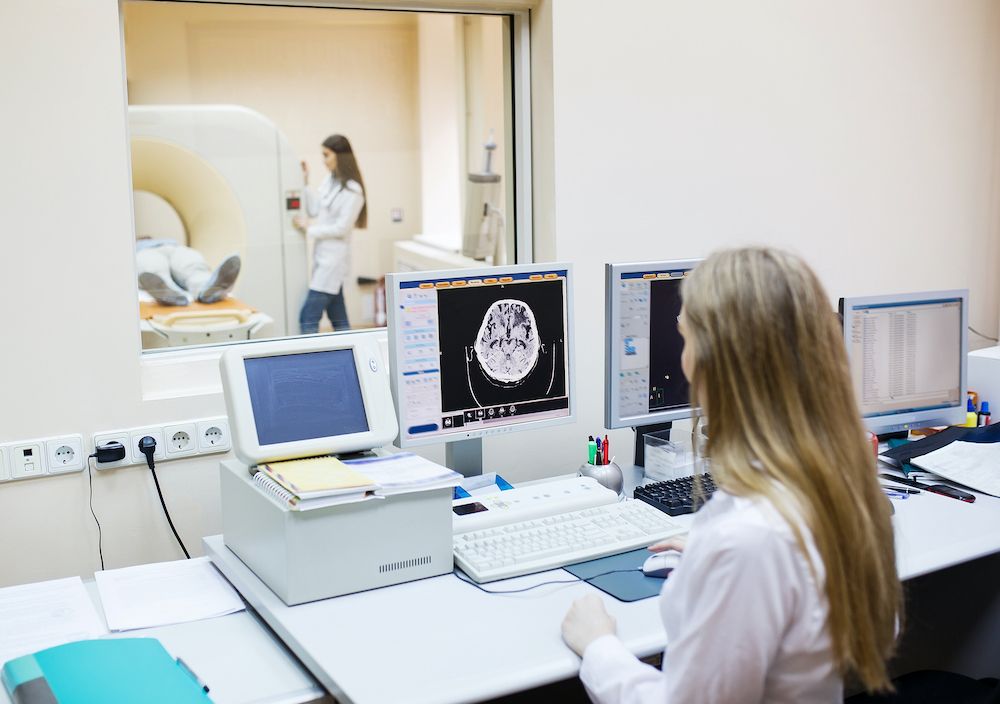
Figure 2. Lead glass is used as viewing windows in X-ray rooms as they can achieve high radiation attenuation while maintaining visibility.
Sources
[1] https://www.assayoffice.co.uk/assets/uploads/Crystal%20Glass%20Factsheet.pdf
[2] Davison, S. and Newton, R.G., 2008. Conservation and restoration of glass. Routledge.
[3] http://ec.europa.eu/DocsRoom/documents/13862/attachments/7/translations/en/renditions/pdf