Polyimide: Properties, Processing, and Applications
Polyimide is a polymer of imide monomers and is sometimes abbreviated as PI. It is regarded as a beneficial engineering plastic because of its unique physio-mechanical and chemical properties. These properties make it suitable for a diverse range of applications, from aerospace to the transportation industries. Marston Bogert, an American Chemist and a former president of IUPAC, successfully produced the first class of polyimides (aromatic polyimide) in 1908. His experiment was successful only after the polycondensation of anhydride of 4-aminophthalic acid. Figure 1 shows the general chemical structure of a polyimide.
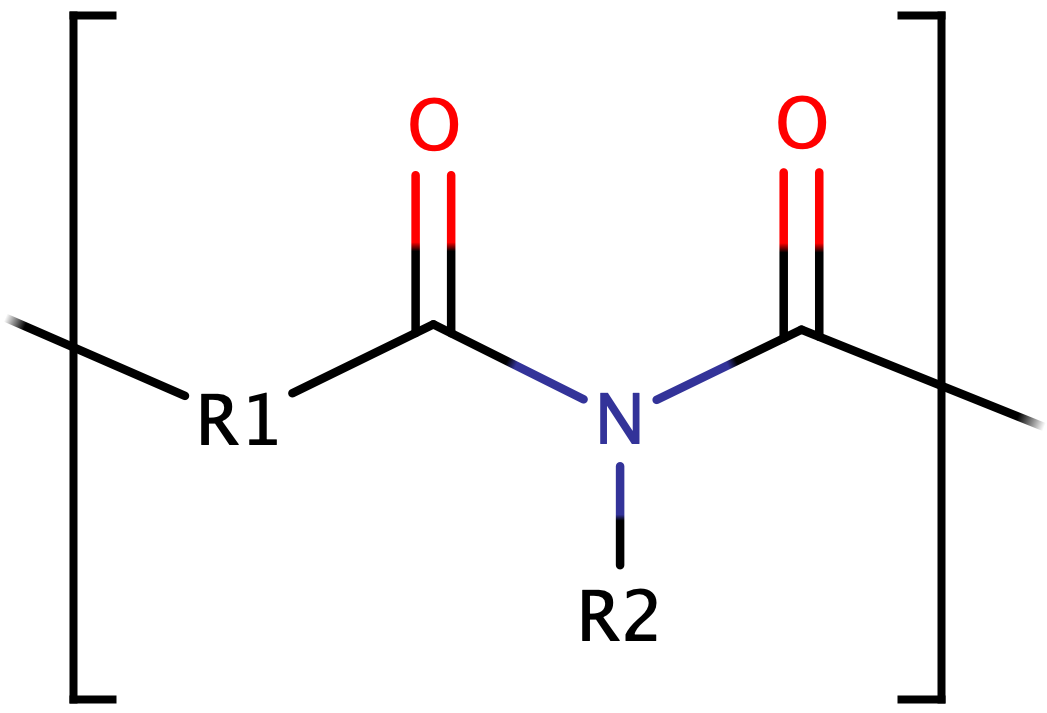
Figure 1. The General Chemical Structure of a Polyimide
Even after the first synthesis of polyimide in the early 20th century, only a few people envisaged the widespread use of the material today. Production started in 1955 through a two-stage polycondensation of pyrometallic dianhydride with diamines. Since then, the yearly production of polyimides has increased drastically, and the interest of researchers has grown exponentially. Here, you will learn about:
- Synthesis of polyimides
- Classification of polyimides
- Properties of Polyimides
- Applications of Polyimides
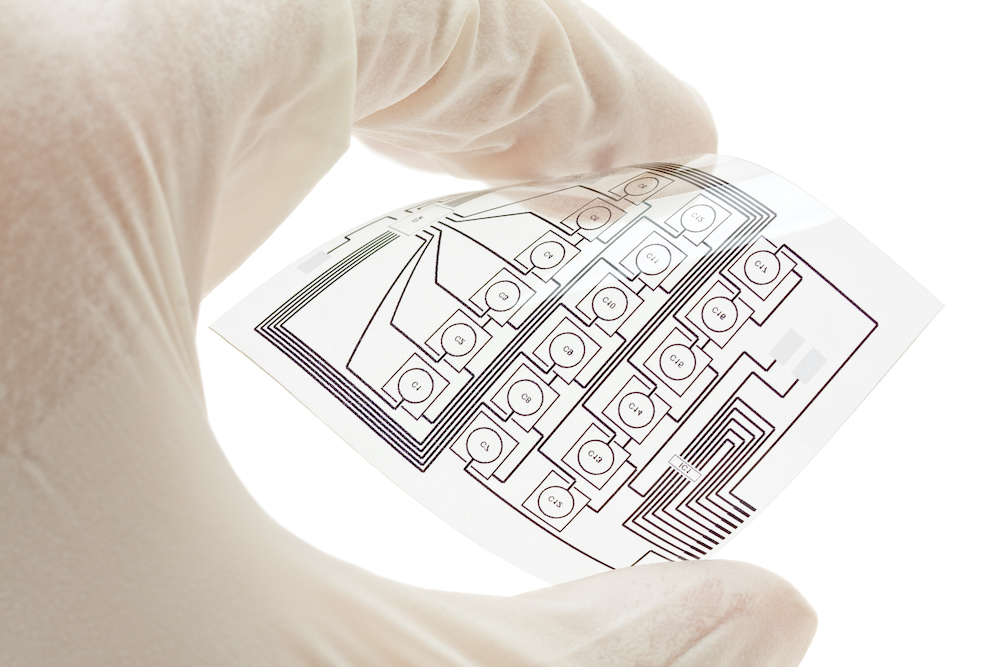
Figure 2. Polyimide is used as a substrate for flexible electronics and flexible solar cells.
Synthesis of Polyimide
The processing of polyimides is quite challenging because of their low solubility in common solvents and their high softening temperature. Even when research proposes a new process to tackle these deficiencies, it is often too expensive to be commercially viable.
Nevertheless, polyimides are generally created from the condensation reaction between a dianhydride and a diamine in a two-stage process [1]. The first stage involves the polycondensation of an aromatic dianhydride with a diamine in a polar solvent at low temperature to produce polyamic acid (a soluble intermediate solution.)
In the second stage, the polyamic acid is processed into polyimides. Different products, such as fibres and films, can be obtained from this stage. Hence, the activities in this stage are crucial to achieving the desired end products. The action of heat or the use of dehydrating agents are essential in converting polyamic acid into fully cyclised polyimide.
Classification of polyimides
Polyimides contain two acyl groups that are bonded to nitrogen. They can be categorised based on:
- The composition of their main chains
- The interaction of their main chains
Classification based on the composition of main chains
Polyimides can be categorised as aliphatic, semi-aromatic or aromatic. The aliphatic polyimides are obtained from the combination of aliphatic dianhydride and diamine, while the aromatic polyimides are derived from aromatic dianhydride and diamine. Polyimides can also be grouped as semi-aromatic when the dianhydride or diamine is aromatic, while the other part is aliphatic.
Classification based on the interaction of main chains
Without going into the intricacies of organic chemistry, it is worth noting that polyimides are generally classified as either being thermosetting or thermoplastic. Polyimides are generally thermosetting polymers; however, thermoplastic polyimides can be obtained by introducing large cyclic side groups to the polymer chain [2].
Polyetherimide (PEI), Polyamideimide (PAI) and Polybenzimidazole (PBI) are thermoplastics that are classified as polyimides because they contain the imide group. For example, PEIs are combinations of polymers consisting of both the polyimide and polyether units in the backbone. Because PEIs and PAIs are produced from aromatic diamines and anhydrides, they are grouped under the aromatic class of polyimides. PBIs are prepared from aromatic tetraamine and an aromatic dicarboxylic acid or its derivative. Hence, they are classified as aromatic.
Properties of polyimides
Different polycondensation reactions produce polyimides with different general, electrical and mechanical properties. Because of polyimide's planar aromatic and hetero-aromatic structures, they are usually processed via the solvent route. This method tackles the insolubility and infusibility drawbacks of polyimides since the reaction between a dianhydride and diamine is done in the presence of a polar aprotic solvent to yield polyamic acid.
In recent times, there has been an immense improvement in the thermal stability of polyimides. This improvement is a result of the incorporation of aromatic rings on the backbone or side rings. These modifications have made the polyimides exhibit an exceptional combination of thermal stability, mechanical toughness and chemical resistance.
The properties of these polymers fall under a range of values. These are presented in the table below.
Table 1. Electrical, mechanical, and thermal properties of polyimides
|
Electrical Properties |
|
|
Dielectric Constant |
3.2 – 4.4 |
|
Dielectric Strength |
33 kV/mm |
|
Electrical Resistivity |
8x1012 – 2x1015 Ωm |
|
Sheet Resistivity |
100 – 10000 Ω/sq |
|
Mechanical Properties |
|
|
Elastic Modulus |
2.9 – 20.15 GPa |
|
Flexural Modulus |
1.1 – 510.53 GPa |
|
Flexural Strength |
23.93 – 275 MPa |
|
Maximum Allowed Stress |
85 – 250 MPa |
|
Tensile Strength |
15.95 – 205 MPa |
|
Thermal Properties |
|
|
Coefficient of Thermal Expansion |
9x10-6 – 6x10-5 K-1 |
|
Heat deflection Temperature |
201 – 413.6 °C |
|
Specific Heat Capacity |
960 – 1300 J(/kg.K) |
|
Thermal Conductivity |
0.31 – 0.52 W/(m.K) |
|
Maximum Service Temperature |
Up to 260 °C |
For instance, the general Polyetherimide(PEI), supplied by Xiamen Keyuan Plastic Co., Ltd has a dielectric constant of 33 kV/mm. It also has a density of 1.27 g/cm3, an elongation of 60% at 20 °C, and a tensile strength of 110 MPa at 20 °C. In addition, it has a heat deflection temperature of 201 °C.
Reinforced Polyetherimide also offers excellent thermal resistance, high strength and stiffness, due to reinforcement with 30% glass fibre, and broad chemical resistance. It has a coefficient of thermal expansion between 2x10-5 and 6x10-5 1/K at 20 °C. Its density varies between 1.49 and 1.51 g/cm³, and it has an elastic modulus between 9 -11 GPa at 20 °C.
Applications of polyimides
Polyimides have a higher mechanical strength, thermal stability, and better insulating properties compared to other polymers. These properties make them suitable for electronic applications in the aerospace and transportation industries. These industries have stringent thermal and mechanical requirements for materials.
Because of polyimide's thermal stability and flexibility, it is often used in flexible solar cells as a dielectric substrate. Some of these properties also contribute to its widespread use in medical devices, such as cardiovascular catheters, push rings and neurological devices.
Figure 3. A flexible polyimide film. Courtesy of DuPontTM.
Sources
[1] V. Peesapati, U. Narasimha Rao and R. Pethrick, "Synthesis and Characterisation of Polyamides and Polyimides from Amino/Methylene bis[Benzenamine] Styryl Pyridines", Polymer International, vol. 43, no. 1, pp. 8-12, 1997.
[2] W. Wright and M. Hallden-Abberton, "Polyimides", Ullmann's Encyclopedia of Industrial Chemistry, 2000.