Polyvinyl Chloride (PVC): Properties, Processing, and Applications
Polyvinyl chloride, commonly referred to as PVC or Vinyl, is the world’s 3rd most synthesised thermoplastic material. Its most well-known application is the formation of PVC pipes in the building and construction industry, but the benefits of PVC extend far beyond this into the medical, electrical and protective clothing sectors.
PVC is the most sought after polymer material in the building and construction industry and currently accounts for 10.2% of the overall European plastic demand, trailing polyethylene (PE) and polypropylene (PP) [1]. This number, however, continues to increase as it looks to replace traditional materials such as wood, metal, concrete and ceramics in a variety of applications.
Here, you will learn about:
- Structure and properties of PVC
- Production and processing of PVC
- Applications of PVC
- Commercial grades of PVC

Properties of PVC
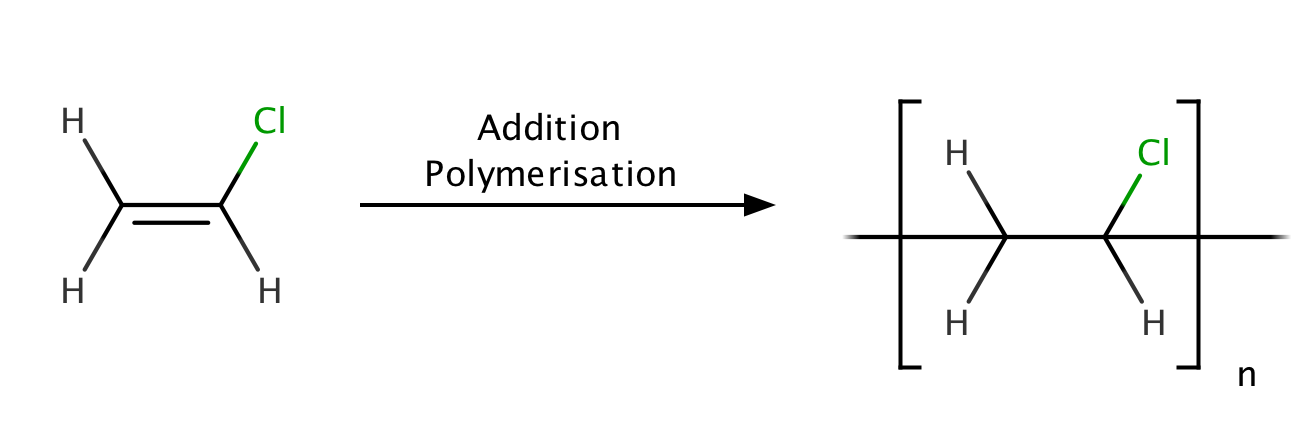
Figure 1. Polyvinyl Chloride structure [2]
PVC is a white, brittle solid available in powder form or granules, formed through an addition polymerisation reaction between vinyl chloride monomers (Figure 1). This solid form can then be modified with the addition of fillers and plasticisers depending on the task at hand. There are, therefore, many different PVC products available with varying additive compositions and properties.

Figure 2. Comparison between the compositions of flexible polyvinyl chloride (Top) and rigid polyvinyl chloride (Bottom) [3]
There are two main classes of PVC (Figure 2):
The addition of plasticisers to PVC acts as a lubricant to the rigid crystalline polymer chains, lowering the crystallinity and yielding a much clearer and flexible plastic material. On the other hand, without the addition of these plasticisers, PVC remains a rigid, stiff material with high resistance to impact, weather, chemicals and corrosive environments. A comparison of some important properties of the two different classes is included in the table below.
Table 1. Comparison between the properties of flexible polyvinyl chloride and rigid polyvinyl chloride [2]
|
Properties |
Plasticised (Flexible) PVC |
Unplasticised (Rigid) PVC |
|
Physical Properties |
||
|
Density |
1.3 – 1.7g/cm3 |
1.35 – 1.5g/cm3 |
|
Glass Transition Temperature |
-5 – -5°C |
60 – 100°C |
|
Mechanical Properties |
||
|
0.001 – 1.8 GPa |
2.4 – 4 GPa |
|
|
0.001 – 1.8 GPa |
2.1 – 3.5 GPa |
|
|
100 – 400% |
25 – 80% |
|
|
Service Temperature |
||
|
Max. Continuous Service Temperature |
50 – 80°C |
50 – 80°C |
|
Min. Continuous Service Temperature |
-40 – -5°C |
-10 – 1°C |
|
Other Properties |
||
|
10 – 30 kV/mm |
10 – 40 kV/mm |
|
|
Transparency |
75 – 85% |
80% |
|
Thermal Insulation (Thermal Conductivity) |
0.16 W/m.K |
0.16 W/m.K |
Production and Processing of PVC
Manufacture of PVC
There are two popular methods for the manufacture of PVC through the addition polymerisation process shown above:
Suspension PVC (S-PVC)
In the suspension process, the obtained PVC particles are mixed with plasticisers and can be then extruded in pellets, which are further used for extrusion, calendering, injection moulding and so on. The equipment necessary for such a process is typically very expensive.
Bulk or emulsion PVC (E-PVC)
In the emulsion process, PVC powder is mixed with plasticisers to produce a paste/resin, which is then used for coatings, dipping and spraying. The initial PVC powder costs more than the particles used in the former process; however, the equipment needed is contrastingly inexpensive.
Processing of PVC
PVC resin obtained from the above processes is extremely unstable due to low thermal stability and high melt viscosity. It needs to be modified before being processed into finished products. Compatible plasticisers can be added as softening agents to enhance some mechanical properties, while fillers can increase the stiffness, impact performance and add colour, opacity and conductivity. Heat stabilisers increase the thermal stability, and lubricants reduce the melt viscosity preventing overheating. The PVC product is then commonly moulded into desired shapes using extrusion, injection moulding and calendering. Resulting products can be PVC films, sheets, boards and tubes.
Chlorinated polyvinyl chloride (CPVC) is manufactured by chlorinating the PVC product, typically increasing its chlorine content from 56% to 66% [2]. This lowers the crystallinity of the polymer, increasing its flexibility and ability to be moulded into useful shapes such as containers and packaging.
PVC Blends
PVC can also be blended with other thermoplastic materials to improve specific properties. Polyester blends combine the excellent processing characteristics of PVC with the superior physical properties of polyesters, increasing abrasion resistance, tensile strength and tear resistance. A polyurethane blend also offers similar results with increased abrasion and chemical resistance. PVC can also be blended with nitrile rubber (NBR), to increase flexibility and elastic recovery properties.
Applications of PVC
Rigid PVC
Not only are these rigid PVC sheets and tubes used commonly for pipes, window frames and roofing produced in the construction industry, but also for much of the safety equipment worn by the construction workers themselves. In the electrical industry, PVC is very useful in insulation pipes, jacketing, switches, plug housings and battery terminals due to its high electrical insulation and dielectric strength.
Flexible PVC
Although flexible PVC also has a range of applications in the construction industry as in flooring, cable insulations, and waterproof linings, its most useful application is in the medical industry. Flexible PVC is used in oxygen tents, gloves, bags and tubing for blood transfusions, drips and dialysis liquids due to its chemical resistance and durability. Some further uses of flexible PVC include waterproof clothing, life-jackets, inflatables and sporting goods.

Sources
[1] PlasticsEurope, “Plastics - the Facts 2018,” 2018. [Online]. Available: https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf
[2] SpecialChem, “Comprehensive Guide on Polyvinyl Chloride (PVC),” 2017. [Online]. Available: https://omnexus.specialchem.com/selection-guide/polyvinyl-chloride-pvc-plastic
[3] KEMI, “Statistics in brief - PVC,” [Online]. Available: https://www.kemi.se/en/statistics/statistics-in-brief/substances-and-substance-groups/pvc