Silicon Carbide: Properties, Production, and Applications
What is silicon carbide (SiC)?
Silicon carbide, with the chemical symbol SiC, is a solid industrial mineral crystalline. It is used as a semiconductor and a ceramic, commonly referred to as carborundum. SiC exists naturally in an extremely rare mineral called moissanite. Pure silicon carbides appear as colourless and transparent crystals. When impurities are added such as nitrogen or aluminium, silicon carbide crystals appear green or blue depending on the level of impurity. Silicon carbide is mostly used for its hardness and strength, though its combined ceramic and semiconductor properties make SiC excellent in the manufacturing of fast, high-voltage, and high-temperature devices [1].
Properties of silicon carbide
Robust crystal structure
Silicon carbide is composed of light elements, silicon (Si) and carbon (C). Its basic building block is a crystal of four carbon atoms forming a tetrahedron, covalently bonded to a single silicon atom at the centre. SiC also exhibits polymorphism as it exists in different phases and crystalline structures [2][3].
High hardness
Silicon carbide has a Mohs hardness rating of 9, making it the hardest available material next to boron carbide (9.5) and diamond (10). It is this apparent property that makes SiC an excellent material choice for mechanical seals, bearings, and cutting tools.
High-temperature resistance
Silicon carbide’s resistance to high temperature and thermal shock is the property that allows SiC to be used in the manufacturing of fire bricks and other refractory materials. The decomposition of silicon carbide starts at 2000°C [2].
Conductivity
If SiC is purified, its behaviour manifests that of an electrical insulator. However, by governing impurities, silicon carbides can exhibit the electrical properties of a semiconductor. For example, introducing varying amounts of aluminium by doping will yield a p-type semiconductor. Typically, an industrial-grade SiC has a purity of about 98 to 99.5%. Common impurities are aluminium, iron, oxygen, and free carbon [2].
Chemical stability
Silicon carbide is a stable and chemically inert substance with high corrosion resistance even when exposed or boiled in acids (hydrochloric, sulphuric, or hydrofluoric acid) or bases (concentrated sodium hydroxides). It is found to react in chlorine, but only at a temperature of 900°C and above. Silicon carbide will start an oxidation reaction in the air when the temperature is at approximately 850°C to form SiO2 [2].
Production of silicon carbide
Silicon carbide can be found in the mineral moissanite, but it is rarely found in nature. So, it is synthetically produced by a synthesising technique called the Acheson method, named after its inventor, Edward G. Acheson. Pure silica (SiO2), or quartz sand, and finely ground petroleum coke (carbon) are mixed and heated in an electric resistive furnace to an elevated temperature of around 1700 to 2500°C. Here below is the main chemical reaction resulting in the formation of ɑ-SiC:
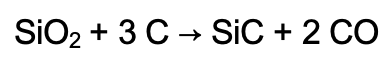
Silicon carbide develops a cylindrical ingot around the core, forming layers of ɑ-SiC, β-SiC, and an unreacted material on the outside. ɑ-SiC is the highest grade with a coarse crystalline structure, and β-SiC is the metallurgical grade. Based on the raw material quality, the SiC can be produced as either green or black. The SiC ingots are then sorted and processed for the specific application they are intended for. They may be crushed, milled, or chemically treated to achieve the properties required for utilisation [4].
Applications of silicon carbide
With the excellent combination of properties that silicon carbide has, it is found to be a promising material option for high-temperature and wear-resistant applications [3].
Abrasive material
Silicon carbide powders are utilised for abrasive machining processes such as grinding, sandblasting, and water-jet cutting. SiC can be laminated in paper, cloth, or wood to produce frictional grip. It can also be used for shaping, honing, and polishing other materials.
High-temperature gas sensor
Silicon carbide is used as a sensing device in chemical production, and in turbine or engine testing industries to detect flammable and combustible gases in harsh, high-temperature, and corrosive environments [3].
Electronics
Silicon carbides are used as semiconductors in many circuit elements due to their high voltage resistance. SiC’s voltage resistance is ten times higher than that of ordinary silicon and even performs better than gallium nitride in systems that exceed 1000V. As such, SiC proves valuable in the development of electric vehicles, solar power inverters, and sensor systems [5].
Sources
[1] G. L. Harris, Properties of Silicon Carbide, London: INSPEC, 1995.
[2] S. Somiya, Y. Inomata, Silicon Carbide Ceramics—1: Fundamental and Solid Reaction, Essex: Elsevier Science Publishers LTD, 2012.
[3] S. E. Saddow, A. Agarwal, Advances in Silicon Carbide Processing and Applications, MA: Artech House Inc, 2004.
[4] “SiC Production Process,” Fiven, n.d., [Online] Available: https://www.fiven.com/world-of-silicon-carbide/sic-production-process/ [Accessed Dec. 11, 2020]
[5] M. R. Nichols, “What are the benefits of silicon carbide in semiconductors?” Euroscientist, March 2019, [Online] Available: https://www.euroscientist.com/what-are-the-benefits-of-silicon-carbide-in-semiconductors/ [Accessed Aug. 30, 2019]