Polyurethane: Properties, Processing, and Applications
Polyurethanes are a large class of polymers that can be tailored to a wide range of applications, making a significant contribution to the construction, automotive and electrical sectors.
Polyurethane is more commonly known for liquid coatings and paints, but applications can also vary from soft, flexible foams to rigid insulation. This broad range of applications is possible as there are both thermoplastic and thermosetting polyurethanes.
Polyurethane was originally synthesised as a substitute for natural rubber in World War II. Shortly after, the versatility of this new polymer and its ability to replace scarce materials gave rise to numerous applications. Nowadays, this group of polymers accounts for 7.7% of European plastic demand, behind commodity polymers polyethylene, polypropylene, and PVC [1].
Here, you will learn about:
- Structure and properties of polyurethane
- Production and processing of polyurethane
- Applications of polyurethane
- Commercial grades of polyurethane

Figure 1. Polyurethane cubes.
Properties of polyurethane
Polyurethane is produced in a polymerisation reaction between diols (or polyols: alcohols with two or more reactive hydroxyl –OH groups) and diisocyanates (or polyisocyanates: isocyanates with two or more reactive isocyanate –NCO groups). The result is a molecule bonded by urethane (COONH) linkages.
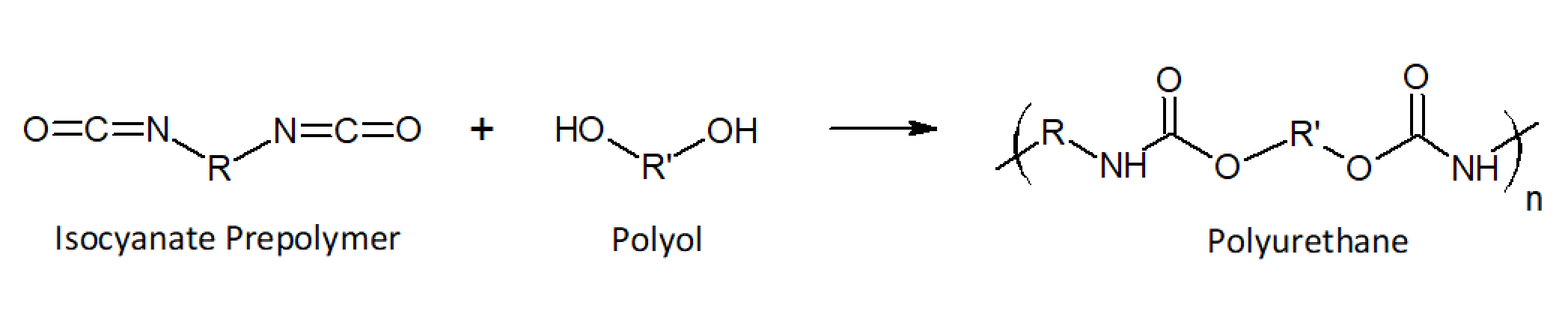
Figure 2. Polyurethane Structure [2].
There are a number of choices of alcohol molecules and corresponding isocyanate molecules, each combination producing a new polyurethane material with new properties. The properties of polyurethanes vary depending on the structure of this polymer backbone and can be tailored to have high strength and rigidity, or high flexibility and toughness.
Thermoplastic Polyurethane vs Thermosetting Polyurethane
The chosen polyol molecule has a large influence on the properties and degree of crosslinking in the polyurethane product. In particular, the number of hydroxyl groups per molecule and the size and flexibility of the hydrocarbon backbone can be chosen to tweak the mechanical properties of the resulting polyurethane material.
If a diol reacts with the diisocyanate, it forms a linear, thermoplastic polymer.
If the alcohol has more than two hydroxyl groups, this will then result in a rigid, cross-linked, thermosetting molecule.
Table 1. Thermoplastic Polyurethane Properties.
|
Properties |
Thermoplastic Polyurethane |
|
Density |
0.05 - 1.7 g/cm3 |
|
0.03 - 1.88 GPa |
|
|
0.029 - 18 GPa |
|
|
2 - 950% |
|
|
45-98 Shore A 51-85 Shore D |
|
|
100-200 10-6 /ºC |
|
|
0.14 - 0.5 W/m.K |
|
|
Max. Service Temperature |
80 - 90°C (120-135°C short-term) |
|
Min. Service Temperature |
~ -60°C |
|
17-25 kV/mm |
Production and processing of polyurethane
Given polyurethanes are created in a reaction between diols and diisocyanates, the manufacturing process can be split into three parts:
- Production of diols
- Production of isocyanates
- Production of polyurethane from these components.
The polyol used in the production of polyurethanes is usually a polyether (in 90% of polyurethanes), or a polyester, with terminal hydroxyl groups. Additionally, there are many aromatic and aliphatic polyisocyanates; however, the most important of these, toluene diisocyanate (TDI) and methylene diphenyl diisocyanate (MDI), contribute to the production of around 95% of all polyurethanes [3]. TDI is generally used in the production of soft, flexible foams for cushioning, whereas MDI is used in the production of more versatile, rigid polyurethanes.
If a diol reacts with either TDI or MDI, this forms a linear, thermoplastic polymer through a condensation polymerisation reaction. If the alcohol has more than two hydroxyl groups, this will result in a rigid, cross-linked, thermosetting molecule.
Additives are commonly added to the mixture to improve certain properties, such as cross-linking agents, chain-extending agents, blowing agents, surfactants, fillers, plasticisers, pigments and flame retardants. Blowing agents will create a polyurethane foam, and surfactants will control the bubble formation and, therefore, the cell formation of the foam. Fillers increase stiffness, plasticisers reduce hardness and pigments add colour to the material.

Figure 3. A handstamp on a polyurethane memory foam mattress after pressing test.
Polyurethane foam
The two reactant liquids combine to form a solid polymer, which may be elastic or rigid. This solid, however, may also contain bubbles making it a cellular foam material. These bubbles can either be formed chemically or physically. Chemical blowing can be achieved by adding water to the polyol, which in turn reacts with the isocyanates to form carbon dioxide gas bubbles. Alternatively, physical blowing is achieved by adding in a substance with a relatively low boiling point, such as pentane. As the exothermic polymerisation reaction proceeds, the pentane heats up and evaporates into bubbles.
This procedure can be controlled depending on the application at hand. For example, a shoe sole may be ‘blown’ to twice its volume, whereas cushions may be blown to 30-40 times the volume. In some low-density foams for cushioning and insulation, only 3% of the total volume is made up of solid polyurethane [3].
Applications of polyurethane
Since there are such a large number of polyisocyanate and polyol substances available for the production of polyurethane, a broad variety of materials can be produced to meet the needs of specific applications. Its relatively lightweight, and its versatility makes it an optimal material for construction, automotive, marine and even apparel applications [4].
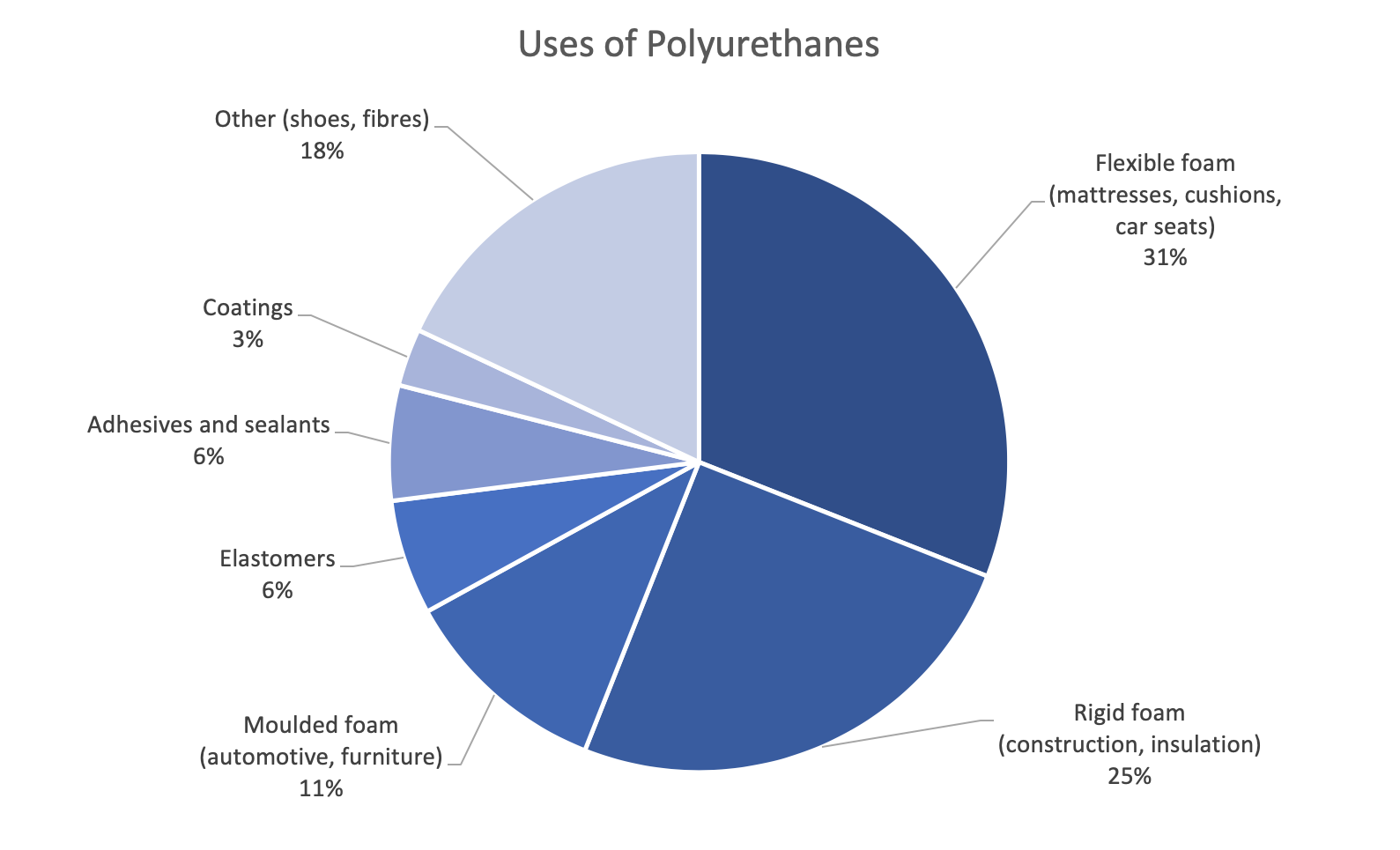
Figure 4. Uses of Polyurethanes (reproduced from Figure 1 in [3])
Flexible Polyurethane Foam
Flexible polyurethane foam is light, durable, supportive and comfortable. It is commonly used for cushioning in bedding, furniture, automotive interiors, carpet underlay and packaging. This accounts for 30% of the polyurethane market due to its commodity usage [5].
Rigid Polyurethane Foam
Rigid polyurethane foams are the most economic and energy-efficient insulations, significantly cutting energy costs. When used in roof and wall insulation, insulated windows and doors, it helps maintain a uniform temperature and reduce noise levels. Rigid polyurethane foam is also commonly used as thermal insulation in refrigerators and freezers.
Coatings, Adhesives, Sealants and Elastomers
Polyurethane coatings can enhance a product’s appearance and increase its lifespan. A polyurethane finish can be used to add shine to the surface of an object, offering relatively better properties than traditional lacquer, shellac and varnish finishes. Wipe-on polyurethane or polyurethane paint is usually an oil-based polyurethane coating applied to wooden or concrete surfaces to add colour and increase durability, as it is usually too thick to spray on. Water-based polyurethane, however, is becoming more popular as it is less toxic and takes less time to dry than its oil-based counterpart [6].
Polyurethane adhesives provide strong bonding advantages, especially soon after it is manufactured, and polyurethane sealants offer tighter seals than traditional counterparts. Polyurethane elastomers can be moulded into any shape required, are lighter than metal, offer increased stress recovery, and are very environmentally resistant.

Figure 5. Polyurethane materials in different shapes and forms.
Sources
[1] Plastics – the Facts 2018, PlasticsEurope [Online].
[2] Polyurethanes, Polymer Database [Online].
[3] Polyurethanes, Essential Chemical Industry [Online].
[4] How Polyurethane is Made, American Chemistry Council [Online].
[5] Polyurethane Applications, American Chemistry Council [Online].
[6] Difference between Polyurethane and Lacquer, Difference Between [Online].